IPCC Special Report on the
Ocean and Cryosphere in a Changing Climate (SROCC)
https://www.ipcc.ch/srocc/
https://www.ipcc.ch/srocc/chapter/chapter-5/
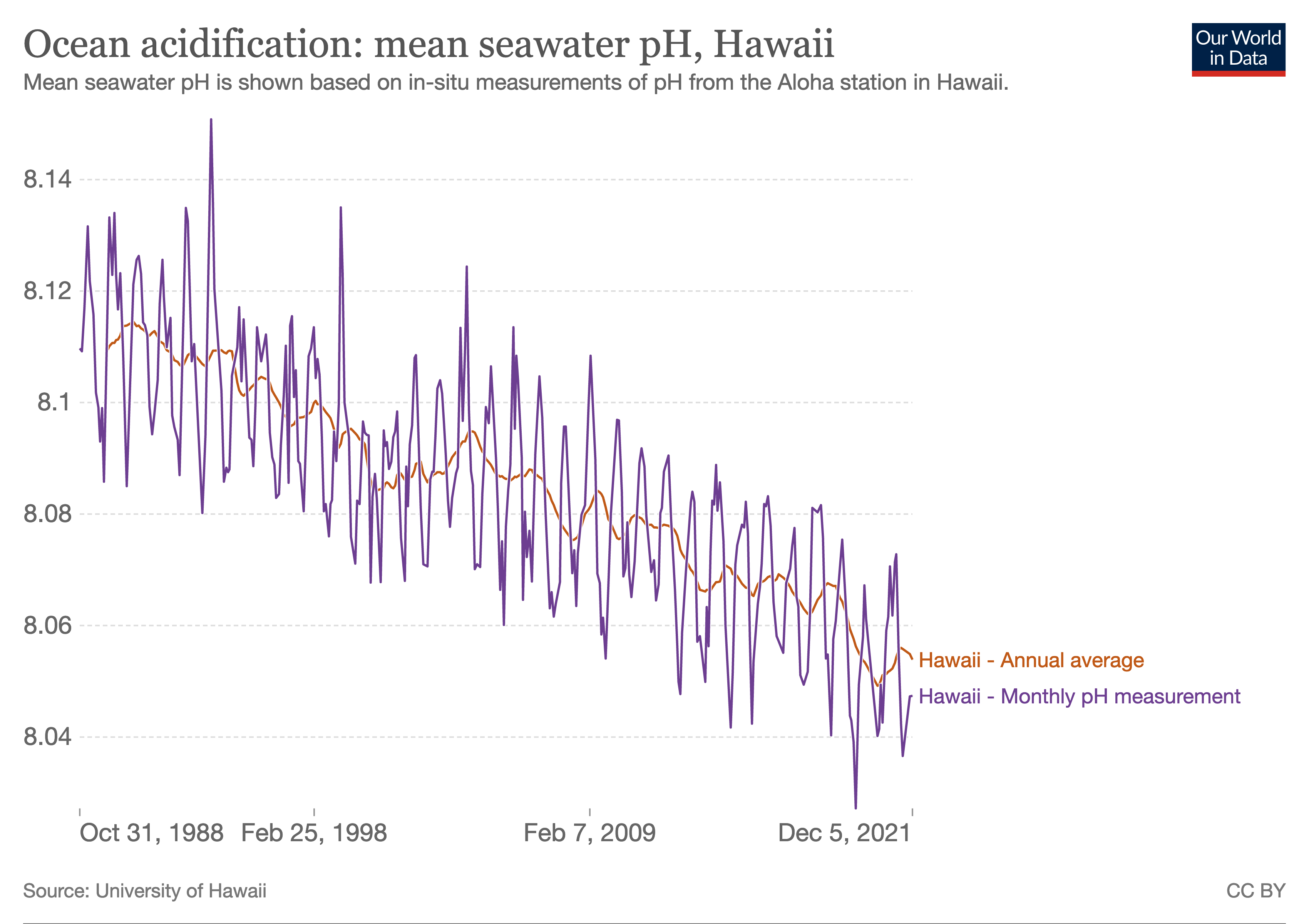
The ocean is continuing to acidify in response to ongoing
ocean carbon uptake. The open ocean surface water pH is
observed to be declining (virtually certain) by a very
likely range of 0.017-0.027 pH units per decade since the
late 1980s across individual time series observations longer
than 15 years. The anthropogenic pH signal is very likely to
have emerged for three-quarters of the near-surface open
ocean prior to 1950 and it is very likely that over 95% of
the near surface open ocean has already been affected. These
changes in pH have reduced the stability of mineral forms of
calcium carbonate due to a lowering of carbonate ion
concentrations, most notably in the upwelling and
high-latitude regions of the ocean.
There is a growing consensus that the open ocean is losing
oxygen overall with a very likely loss of 0.5-3.3% between
1970-2010 from the ocean surface to 1000 m (medium
confidence). Globally, the oxygen loss due to warming is
reinforced by other processes associated with ocean physics
and biogeochemistry, which cause the majority of the
observed oxygen decline (high confidence). The oxygen
minimum zones (OMZs) are expanding by a very likely range of
3-8%, most notably in the tropical oceans, but there is
substantial decadal variability that affects the attribution
of the overall oxygen declines to human activity in tropical
regions (high confidence).
Ocean Acidification (Wikipedia)
https://en.wikipedia.org/wiki/Ocean_acidification
 Of the extra carbon dioxide (CO2) added into the oceans,
some remains as dissolved carbon dioxide (CO2), while the rest
contributes towards making additional bicarbonate (HCO3-)
and additional carbonic acid (H2CO3). This also increases the
concentration of hydrogen ions (H+).
Many ocean plants and animals build shells and skeletons out
of two chemicals that exist in seawater, calcium (Ca) and
carbonate (CO3). Organisms combine calcium and carbonate to
form hard shells and skeletons out of the mineral calcium
carbonate. Therefore, the plants and animals that use
calcium carbonate for structure and protection are called
calcifying organisms. Increased acidity SLOWS the growth of
calcium carbonate structures, and under severe conditions,
can dissolve structures faster than they form.
Of the extra carbon dioxide (CO2) added into the oceans,
some remains as dissolved carbon dioxide (CO2), while the rest
contributes towards making additional bicarbonate (HCO3-)
and additional carbonic acid (H2CO3). This also increases the
concentration of hydrogen ions (H+).
Many ocean plants and animals build shells and skeletons out
of two chemicals that exist in seawater, calcium (Ca) and
carbonate (CO3). Organisms combine calcium and carbonate to
form hard shells and skeletons out of the mineral calcium
carbonate. Therefore, the plants and animals that use
calcium carbonate for structure and protection are called
calcifying organisms. Increased acidity SLOWS the growth of
calcium carbonate structures, and under severe conditions,
can dissolve structures faster than they form.
 Changes in ocean chemistry can have extensive direct and
indirect effects on organisms and their habitats. One of the
most important repercussions of increasing ocean acidity
relates to the production of shells and plates out of
calcium carbonate (CaCO3).
This process is called calcification and is important to the
biology and survival of a wide range of marine organisms.
Calcification involves the precipitation of dissolved ions
into solid CaCO3 structures. After they are formed, such
structures are vulnerable to dissolution unless the
surrounding seawater contains saturating concentrations of
carbonate ions (CO32-).
2010 TED Talk (18 Minutes)
Rob Dunbar: Discovering Ancient Climates in Oceans and Ice
https://www.ted.com/talks/rob_dunbar_discovering_ancient_climates_in_oceans_and_ice
Rob_Dunbar.mp4
Rob Dunbar hunts for data on our climate from 12,000 years
ago, finding clues inside ancient seabeds and corals and
inside ice sheets. His work is vital in setting baselines
for fixing our current climate -- and in tracking the rise
of deadly ocean acidification.
2019 TED Talk (13 Minutes)
What ocean microbes reveal about the changing climate | Angelicque White
https://www.ted.com/talks/angelicque_white_what_ocean_microbes_reveal_about_the_changing_climate
2019s-angelicque-white-004-5000k.mp4
The rate of increase in CO2 is unprecedented for our planet.
Changes in ocean chemistry can have extensive direct and
indirect effects on organisms and their habitats. One of the
most important repercussions of increasing ocean acidity
relates to the production of shells and plates out of
calcium carbonate (CaCO3).
This process is called calcification and is important to the
biology and survival of a wide range of marine organisms.
Calcification involves the precipitation of dissolved ions
into solid CaCO3 structures. After they are formed, such
structures are vulnerable to dissolution unless the
surrounding seawater contains saturating concentrations of
carbonate ions (CO32-).
2010 TED Talk (18 Minutes)
Rob Dunbar: Discovering Ancient Climates in Oceans and Ice
https://www.ted.com/talks/rob_dunbar_discovering_ancient_climates_in_oceans_and_ice
Rob_Dunbar.mp4
Rob Dunbar hunts for data on our climate from 12,000 years
ago, finding clues inside ancient seabeds and corals and
inside ice sheets. His work is vital in setting baselines
for fixing our current climate -- and in tracking the rise
of deadly ocean acidification.
2019 TED Talk (13 Minutes)
What ocean microbes reveal about the changing climate | Angelicque White
https://www.ted.com/talks/angelicque_white_what_ocean_microbes_reveal_about_the_changing_climate
2019s-angelicque-white-004-5000k.mp4
The rate of increase in CO2 is unprecedented for our planet.
Ocean Temperature
Daily Sea Surface Temperature (Interactive)
https://climatereanalyzer.org/clim/sst_daily/?dm_id=world2
Warming Lakes and Oceans can't hold as much Oxygen
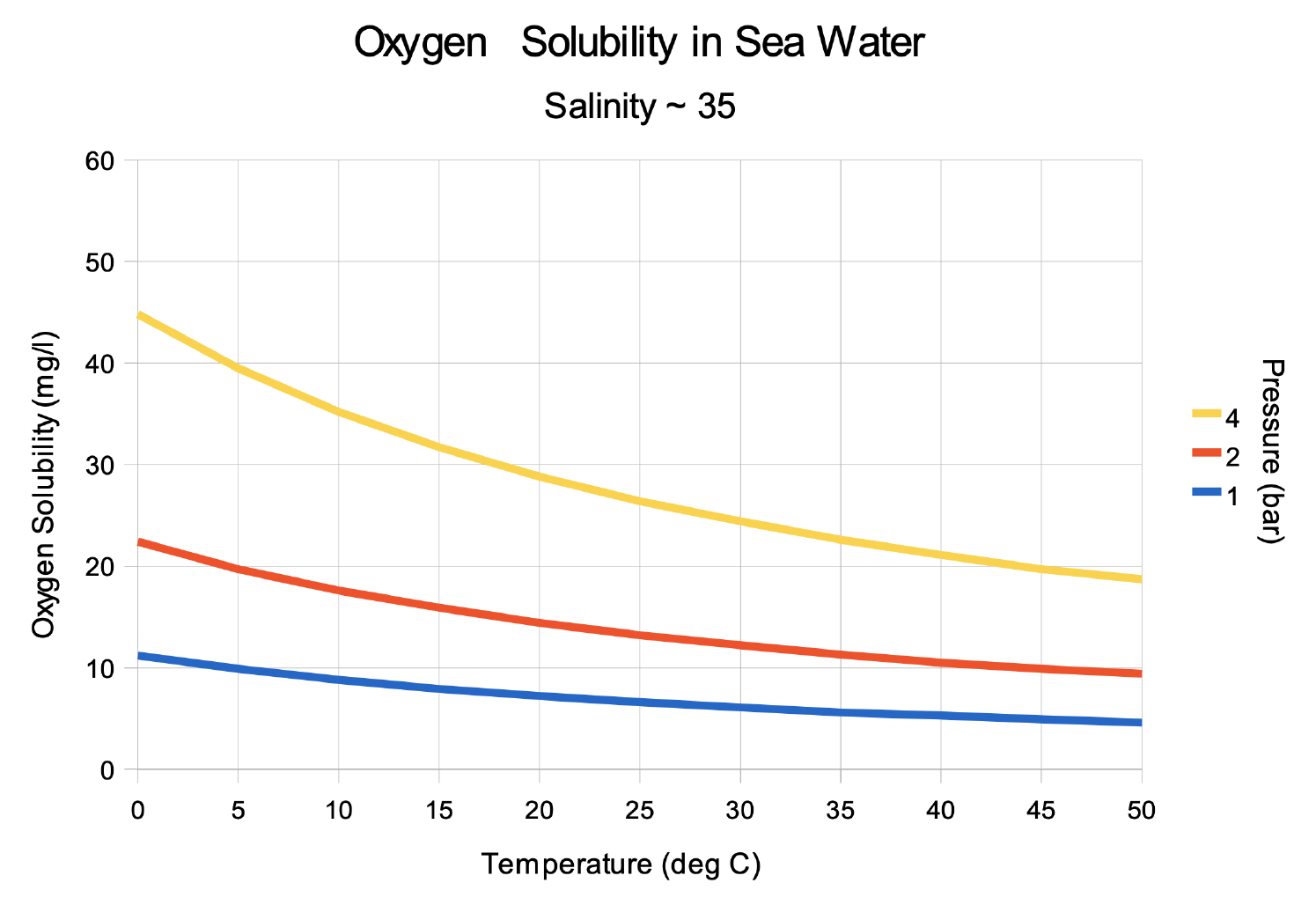 10, 20, and 40 meters of Depth
How Earth’s Biggest Mass Extinctions Stack Up
https://www.scientificamerican.com/article/how-earths-biggest-mass-extinctions-stack-up/
Earth has experienced five major mass extinctions, with the
most lethal occurring around 252 million years ago, likely
caused by volcanism and its subsequent effects. While
volcanism is a common trigger, the actual kill mechanisms,
such as ocean anoxia and ozone depletion, vary. Studying
these past events provides valuable insights into the
potential impending biodiversity crisis of the Anthropocene
age.
10, 20, and 40 meters of Depth
How Earth’s Biggest Mass Extinctions Stack Up
https://www.scientificamerican.com/article/how-earths-biggest-mass-extinctions-stack-up/
Earth has experienced five major mass extinctions, with the
most lethal occurring around 252 million years ago, likely
caused by volcanism and its subsequent effects. While
volcanism is a common trigger, the actual kill mechanisms,
such as ocean anoxia and ozone depletion, vary. Studying
these past events provides valuable insights into the
potential impending biodiversity crisis of the Anthropocene
age.
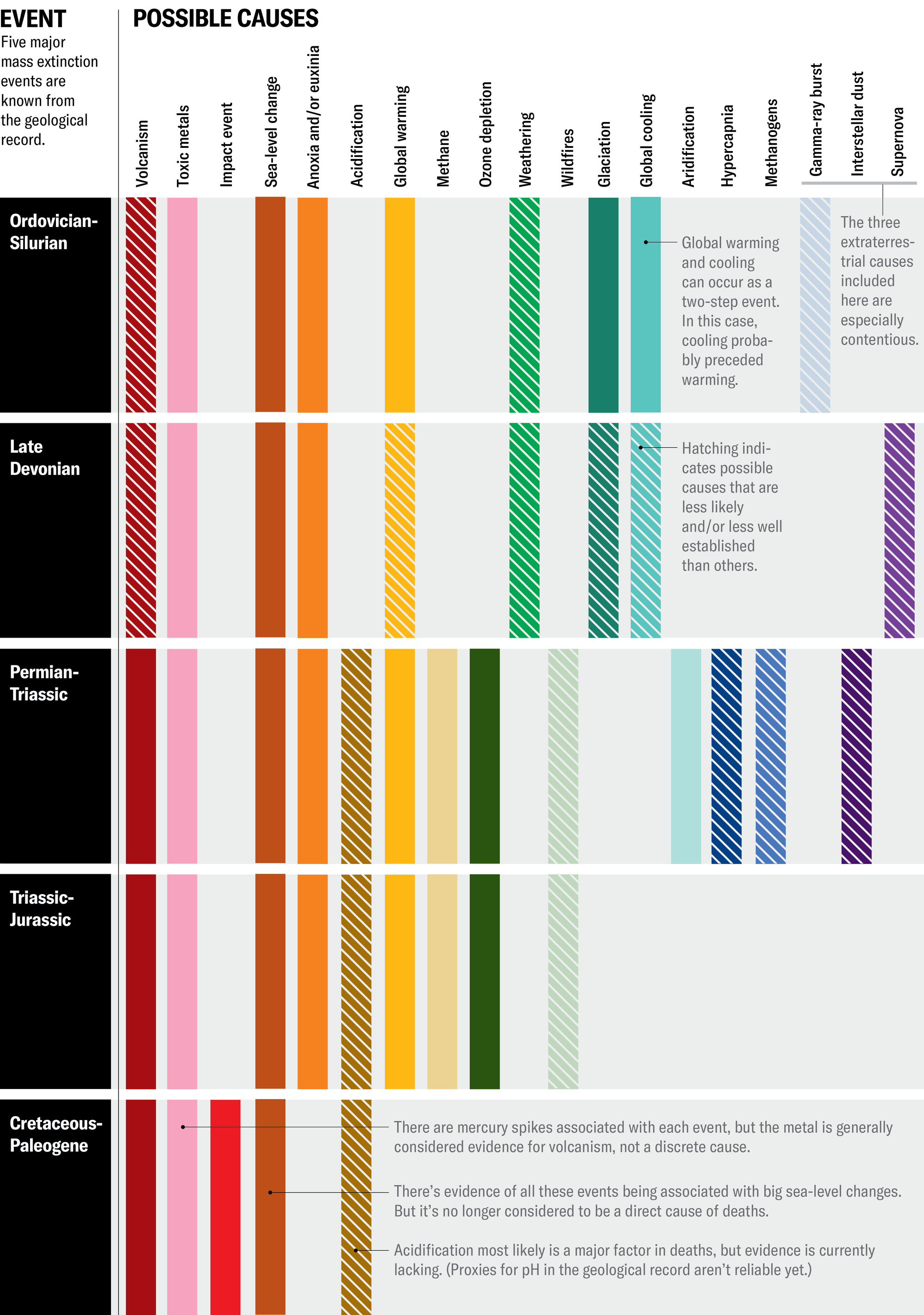 NOVA scienceNOW | Mass Extinction (6- Min)
NOVA scienceNOW: Mass Extinction from 2006
https://www.pbs.org/wgbh/nova/teachers/viewing/3318_01_nsn.html
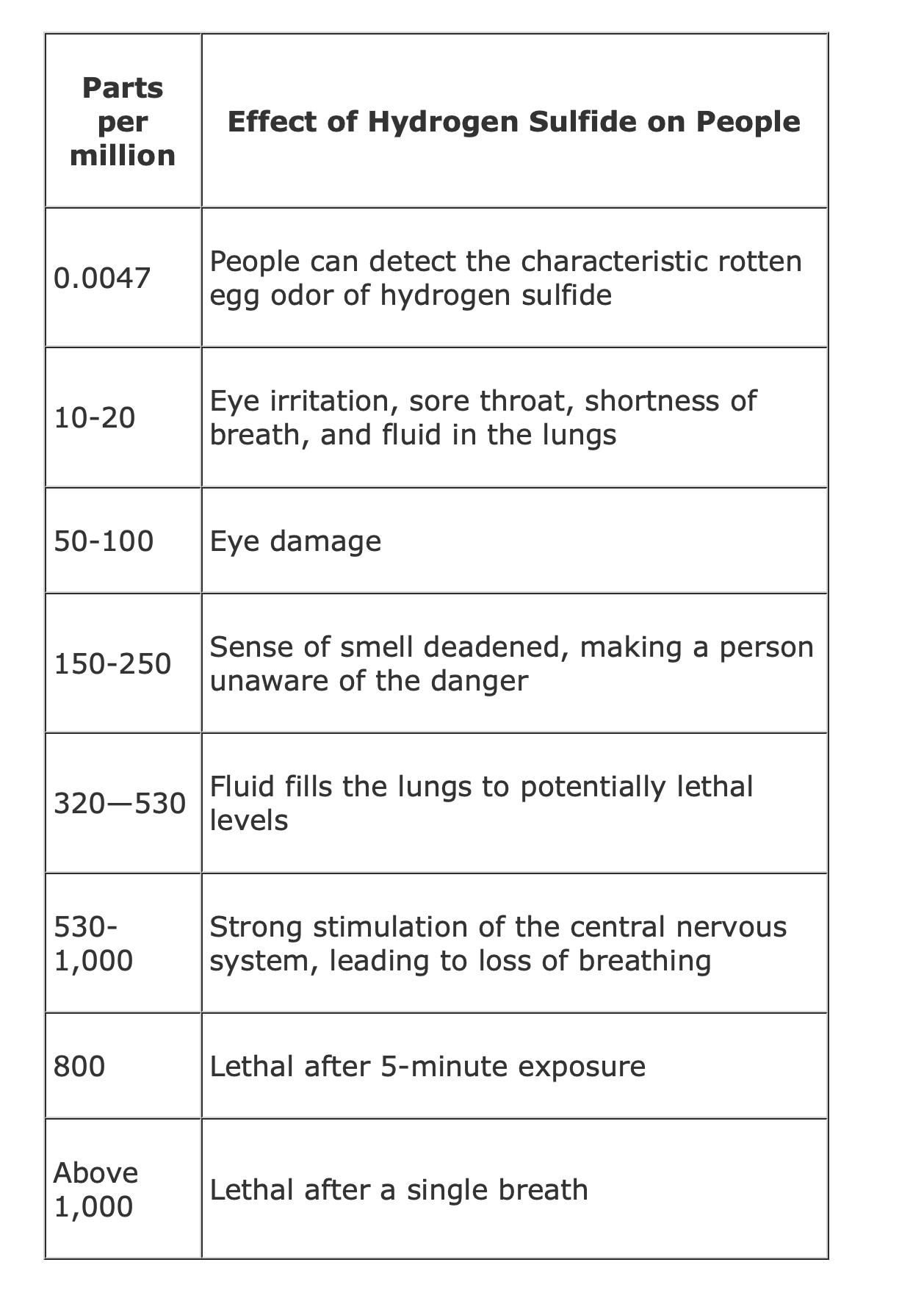
Hydrogen Sulfide (H2S) Toxicity
NOVA scienceNOW | Mass Extinction (6- Min)
NOVA scienceNOW: Mass Extinction from 2006
https://www.pbs.org/wgbh/nova/teachers/viewing/3318_01_nsn.html
Hydrogen Sulfide (H2S) Toxicity
 Five [Clinate Related] Mass Extinctions (excludes the
Cretaceous-Tertiary Mass Extinction due to impact)
https://phys.org/news/2017-06-mass-extinctionsand-planet-today.html
The Late Permian mass extinction around 252m years ago
dwarfs all the other events, with about 96% of species
becoming extinct. This included more trilobites, corals, and
whole branches of species of terrestrial animals. The
extinction was triggered by a vast eruption of the Siberian
Traps, a gigantic and prolonged volcanic event that covered
much of modern day Siberia, which led to a cascade of
environmental effects.
A greenhouse effect rapidly took hold in the atmosphere,
while the oceans suffered acidification and oxygen
depletion. The ozone layer was partially destroyed, meaning
lethal levels of UV radiation reached the Earth's surface.
The recovery took almost 10m years and even then, the
unstable environment this catastrophic crisis created meant
the subsequent Triassic period saw intermittent bursts of
heightened extinction.
So, are we currently in the middle of a mass extinction? If
we really are, this time the cause is not a meteorite impact
or volcanic eruptions. It is the work of a single species:
Homo sapiens. Habitat destruction and climate change from
rising carbon dioxide levels has driven extinction rates to
levels reminiscent of the mass extinctions of the ancient
past.
The similarities between today and the past are uncanny. The
majority of past extinctions are associated with carbon
dioxide from volcanoes causing rapid global warming, which
led to a number of environmental cascade effects. The cause
may be different, but the results will be the same.
sam.wormley@icloud.com
Five [Clinate Related] Mass Extinctions (excludes the
Cretaceous-Tertiary Mass Extinction due to impact)
https://phys.org/news/2017-06-mass-extinctionsand-planet-today.html
The Late Permian mass extinction around 252m years ago
dwarfs all the other events, with about 96% of species
becoming extinct. This included more trilobites, corals, and
whole branches of species of terrestrial animals. The
extinction was triggered by a vast eruption of the Siberian
Traps, a gigantic and prolonged volcanic event that covered
much of modern day Siberia, which led to a cascade of
environmental effects.
A greenhouse effect rapidly took hold in the atmosphere,
while the oceans suffered acidification and oxygen
depletion. The ozone layer was partially destroyed, meaning
lethal levels of UV radiation reached the Earth's surface.
The recovery took almost 10m years and even then, the
unstable environment this catastrophic crisis created meant
the subsequent Triassic period saw intermittent bursts of
heightened extinction.
So, are we currently in the middle of a mass extinction? If
we really are, this time the cause is not a meteorite impact
or volcanic eruptions. It is the work of a single species:
Homo sapiens. Habitat destruction and climate change from
rising carbon dioxide levels has driven extinction rates to
levels reminiscent of the mass extinctions of the ancient
past.
The similarities between today and the past are uncanny. The
majority of past extinctions are associated with carbon
dioxide from volcanoes causing rapid global warming, which
led to a number of environmental cascade effects. The cause
may be different, but the results will be the same.
sam.wormley@icloud.com
Of the extra carbon dioxide (CO2) added into the oceans, some remains as dissolved carbon dioxide (CO2), while the rest contributes towards making additional bicarbonate (HCO3-) and additional carbonic acid (H2CO3). This also increases the concentration of hydrogen ions (H+). Many ocean plants and animals build shells and skeletons out of two chemicals that exist in seawater, calcium (Ca) and carbonate (CO3). Organisms combine calcium and carbonate to form hard shells and skeletons out of the mineral calcium carbonate. Therefore, the plants and animals that use calcium carbonate for structure and protection are called calcifying organisms. Increased acidity SLOWS the growth of calcium carbonate structures, and under severe conditions, can dissolve structures faster than they form.
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells and plates out of calcium carbonate (CaCO3). This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO3 structures. After they are formed, such structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32-). 2010 TED Talk (18 Minutes) Rob Dunbar: Discovering Ancient Climates in Oceans and Ice https://www.ted.com/talks/rob_dunbar_discovering_ancient_climates_in_oceans_and_ice Rob_Dunbar.mp4 Rob Dunbar hunts for data on our climate from 12,000 years ago, finding clues inside ancient seabeds and corals and inside ice sheets. His work is vital in setting baselines for fixing our current climate -- and in tracking the rise of deadly ocean acidification. 2019 TED Talk (13 Minutes) What ocean microbes reveal about the changing climate | Angelicque White https://www.ted.com/talks/angelicque_white_what_ocean_microbes_reveal_about_the_changing_climate 2019s-angelicque-white-004-5000k.mp4 The rate of increase in CO2 is unprecedented for our planet.
10, 20, and 40 meters of Depth How Earth’s Biggest Mass Extinctions Stack Up https://www.scientificamerican.com/article/how-earths-biggest-mass-extinctions-stack-up/ Earth has experienced five major mass extinctions, with the most lethal occurring around 252 million years ago, likely caused by volcanism and its subsequent effects. While volcanism is a common trigger, the actual kill mechanisms, such as ocean anoxia and ozone depletion, vary. Studying these past events provides valuable insights into the potential impending biodiversity crisis of the Anthropocene age.
NOVA scienceNOW | Mass Extinction (6- Min) NOVA scienceNOW: Mass Extinction from 2006 https://www.pbs.org/wgbh/nova/teachers/viewing/3318_01_nsn.html Hydrogen Sulfide (H2S) Toxicity
Five [Clinate Related] Mass Extinctions (excludes the Cretaceous-Tertiary Mass Extinction due to impact) https://phys.org/news/2017-06-mass-extinctionsand-planet-today.html The Late Permian mass extinction around 252m years ago dwarfs all the other events, with about 96% of species becoming extinct. This included more trilobites, corals, and whole branches of species of terrestrial animals. The extinction was triggered by a vast eruption of the Siberian Traps, a gigantic and prolonged volcanic event that covered much of modern day Siberia, which led to a cascade of environmental effects. A greenhouse effect rapidly took hold in the atmosphere, while the oceans suffered acidification and oxygen depletion. The ozone layer was partially destroyed, meaning lethal levels of UV radiation reached the Earth's surface. The recovery took almost 10m years and even then, the unstable environment this catastrophic crisis created meant the subsequent Triassic period saw intermittent bursts of heightened extinction. So, are we currently in the middle of a mass extinction? If we really are, this time the cause is not a meteorite impact or volcanic eruptions. It is the work of a single species: Homo sapiens. Habitat destruction and climate change from rising carbon dioxide levels has driven extinction rates to levels reminiscent of the mass extinctions of the ancient past. The similarities between today and the past are uncanny. The majority of past extinctions are associated with carbon dioxide from volcanoes causing rapid global warming, which led to a number of environmental cascade effects. The cause may be different, but the results will be the same. sam.wormley@icloud.com